Carlos D. Esteban, Kerstin Mahr, Vicente Monedero, Wolfgang Hillen, Gaspar Perez-Martinez and Fritz Titgemeyer.
Published in Microbiology (2004) 150 (3):613-620.
Pubmed link: http://www.ncbi.nlm.nih.gov/pubmed/14993310. Full text:
ABSTRACT :
In low-GC Gram-positive bacteria, the regulatory protein CcpA has been shown to play a major part in the so-called carbon catabolite repression (CCR) process, as well as in the induction of basic metabolic genes, for which it is considered a global regulator. A strain of Lactobacillus casei that carried a complete deletion of ccpA has been constructed and used to test CCR on N-acetyl-glucosaminidase activity and growth performance of a collection of seven CcpA mutations obtained by site-directed mutagenesis. The replaced amino acids were located in the DNA and cofactor (P-Ser-HPr) binding domains. Mutations in the DNA binding domain lacked CCR, as found in Bacillus megaterium. However, mutations in the cofactor binding domain of L. casei CcpA had a different phenotype to that observed in the previous studies with B. megaterium. Two of them, S80L and T307I, displayed a significant hyper-repression, an effect never reported before for CcpA. Comparison of growth capabilities provided by the different mutants and their ability to sustain CCR demonstrated that CCR, at least on the enzymatic activity tested, and growth defect of CcpA mutations are unrelated features.
INTRODUCTION:
In the industrially relevant lactic acid bacterium Lactobacillus casei, the preferential utilisation of carbon sources is controlled by the mechanism of carbon catabolite repression (CCR) (Monedero et al., 1997). As in other low-G+C Gram-positive bacteria, CCR takes place through the binding of the transcriptional repressor CcpA (Miwa et al., 1994; Hueck et al., 1995; Egeter & Brückner, 1996; Lokman et al., 1997; Leboeuf et al., 2000) to an operator sequence called cre (catabolite responsive element) (Fujita et al., 1995; Aung-Hilbrich et al., 2002). CcpA binding to cre sequences is markedly enhanced by its co-repressor, the Ser46 phosphorylated HPr protein (P-Ser-HPr), a key component of the phosphoenolpyruvate-dependent phosphotransferase system (PTS) (Deutscher et al., 1995; Jones et al., 1997). This links CCR to the sugar transport process via the PTS (Brückner & Titgemeyer, 2002). Some small molecules, such as fructose-1,6-bisP, glucose-6-P or NADP, can also influence the interaction between CcpA and cre (Gosseringer et al, 1997; Kim et al., 1998). Therefore, the major role attributed to CcpA was related to CCR in the presence of rapidly metabolisable carbon sources.
CcpA inactivation pleiotropically affects the expression of approximately 8% of the genes in Bacillus subtilis (Moreno et al., 2001). Genes of very important metabolic pathways are regulated by CcpA, such as, citB and citZ, contributing to the Krebs cycle (Kim et al., 2002), gltAB for ammonium assimilation (Faires et al., 1999) or the ilv-leu operon for branched chain amino acid biosynthesis (Ludwig et al., 2002). Other studies revealed that CcpA in Gram-positives also regulates carbon catabolite activation (CCA) of acetoin secretion, acetate biosynthesis genes in B. subtilis (Turinsky et al., 2000) and glycoytic genes in B. subtilis, Lactococcus lactis and Enterococcus faecalis (Luesink et al., 1998; Leboeuf et al., 2000; Ludwig et al., 2001). However, the number of genes subject to CCA could be significantly lower than those under CCR (Leboeuf et al., 2000; Moreno et al., 2001; Titgemeyer & Hillen, 2002).
However, CcpA has also been found to regulate other processes, for example, biofilm formation is subject to CcpA-mediated CCR in B. subtilis, while intact CcpA was required for a full biofilm formation in S. mutants (Wen & Burne, 2002; Stanley et al., 2003). Also transcription of the capsular polysaccharide biosynthesis locus (cps) was significantly reduced by mutation of regM (ccpA homologue) in Streptococcus pneumoniae (Giammarinaro & Paton, 2002). Its involvement in such diverse processes made researchers in the field consider CcpA as a global regulator. As consequence, inactivation of ccpA is known to have a strong incidence on growth rate (Miwa et al., 1994; Hueck et al., 1995; Monedero et al., 1997; Leboeuf et al., 2000). This growth defect in B. subtilis has been proposed to be related to lack of expression of the above mentioned gltAB operon, encoding glutamate synthase, necessary for ammonium assimilation (Faires et al., 1999) and the ilv-leu operon (Ludwig et al., 2002).
Four single mutations in CcpA were described in B. megaterium that showed independent effects on growth and CCR (Küster et al., 1999a): three mutations showed no CCR but normal growth and one was solely defective in growth. All of these mutations are located within the N-terminal DNA binding domain of CcpA. Five additional mutations showed glucose-independent CCR and were located in the co-repressor binding domain (Küster et al.,1999b).
The process leading to growth depression has not been studied in lactobacilli up to date. Therefore, during this work equivalent mutations were obtained in Lactobacillus casei CcpA to elucidate if there was similarity in the role of these residues/domains between these species.
MATERIALS AND METHODS:
Bacterial strains, plasmids and grow conditions.
The strains and plasmids used in this work are listed in Table 1. L. casei cells were grown in MRS medium (Oxoid), or MRS basal medium supplemented with 0.5% (w/v) of the appropriate sugar, at 37ºC under static conditions. Escherichia coli was grown with shaking at 37ºC in Luria-Bertani medium. Plating of bacteria was performed on the same media with 1.5% agar. When required, the concentrations of antibiotics used were 100 mg of ampicillin per ml for E. coli and 5 mg of erythromycin or 5 mg of chloramphenicol per ml for L. casei.
Table 1. Strains and plasmids used in this study.
| Strain or plasmid | Description | Source or reference |
| Strains | ||
| L. casei BL23 | ATCC 393 [pLZ15–] | B. Chassy (University of Illinois, Urbana) |
| L. casei BL71 | L. casei BL23 ccpA::erm | (Monedero et al., 1997) |
| L. casei BL190 | L. casei BL23 DccpA | This work |
| E. coli DH5a | general cloning host | (Sambrook et al., 1989) |
| Plasmids | ||
| pCCPA2.6 | pACYC184 plus a 2.6 fragment containing ccpA | Monedero et al., 1997 |
| pUCm1 | pUC19 plus Cmr from pC194 | Monedero et al., 1997 |
| pDccpA | pUCm1 plus up and down ccpA regions | This work |
| pUCCPA | pUC19 plus ccpA | This work |
| pUCCPA-T7S | pUCCPA derivative (codon 7 of ccpA is AGC for Ser) | This work |
| pUCCPA-R50H | pUCCPA derivative (codon 50 of ccpA is CAT for His) | This work |
| pUCCPA-N52S | pUCCPA derivative (codon 52 of ccpA is AGC for Ser) | This work |
| pUCCPA-F78C | pUCCPA derivative (codon 78 of ccpA is TGC for Cys) | This work |
| pUCCPA-S80L | pUCCPA derivative (codon 80 of ccpA is CTC for Leu) | This work |
| pUCCPA-M283V | pUCCPA derivative (codon 283 of ccpA is GTG for Val) | This work |
| pUCCPA-T307I | pUCCPA derivative (codon 307 of ccpA is ATC for Ile) | This work |
| pGAL9 | Err | Pérez-Martínez et al., 1992 |
| pGCCPA | pGAL9 derivative expressing CcpA | Monedero et al., 1997 |
| pGCCPA-T7S | pGAL9 derivative expressing CcpAT7S | This work |
| pGCCPA-R50H | pGAL9 derivative expressing CcpAR50H | This work |
| pGCCPA-N52S | pGAL9 derivative expressing CcpAN52S | This work |
| pGCCPA-F78C | pGAL9 derivative expressing CcpAF78C | This work |
| pGCCPA-S80L | pGAL9 derivative expressing CcpAS80L | This work |
| pGCCPA-M283V | pGAL9 derivative expressing CcpAM283V | This work |
| pGCCPA-T307I | pGAL9 derivative expressing CcpAT307I | This work |
Recombinant DNA procedures.
Restriction and modifying enzymes were used according to the recommendations of manufacturers. General cloning procedures in E. coli were performed as described by Sambrook et al. (1989). Genomic DNA from Lactobacillus casei was purified using Purogene DNA isolation Kit (Gentra Systems), following the procedure described by the manufacturer. L. casei ccpA gene was amplified by PCR with the primers CCPA8 (5’-CGTTGCACTTATCTAGACAATTCG-3’) and CCPA9 (5’-TCAGATCTAAGGAGGAAATCAAATGG-3’) from chromosomal DNA of L. casei. The resulting 1.1 kb fragment was digested with the restriction enzymes BglII and XbaI and ligated to pUC19, previously cleaved with BamHI and XbaI, to obtain plasmid pUCCPA. In order to obtain constitutive expression in L. casei, ccpA was excised from pUCCPA with SmaI and XbaI and it was subcloned into pGAL9 (Pérez-Martínez et al., 1992) digested with BamHI –made blunt with the klenow fragment- and XbaI, rendering plasmid pGCCPA. This allowed to remove the a-amylase gene from pGAL9 and allowed the expression of ccpA in L. casei from the AL9 promoter (Monedero et al., 1997). L. casei was transformed by electroporation with a Gene-pulser apparatus (Bio-Rad Laboratories, Richmond, Calif.) as previously described (Posno et al., 1991). For Southern blot hybridisation, L. casei DNA was digested with EcoRI endonuclease, separated on agarose gel and blotted to a Hybond nylon membrane (Amersham). The probe used in the Southern and dot blot hybridisation experiments consists of an 887 bp fragment of the ccpA gene. This DNA fragment was obtained by PCR using pCCPA2.6 as a template and the oligonucleotides CCPA2 (5’-AAGTAAGTTGTGGCCGAGTCA-3’) and CCPA9 as primers. The probe was prepared using the reagents from the Boerhinger digoxigenin-DNA labelling kit as recommended by the manufacturer. Hybridisation, washing, and staining were done as described by the supplier.
Site directed mutagenesis.
Construction of ccpA mutant alleles was carried out by site-directed mutagenesis following the protocol of Landt et al. (1990). Oligonucleotides for site-specific mutagenesis of ccpA (5’-CACGCATAAATGCTAATTGTTTGC-3’, 5’-CTGCATTCGGATGATAATCAAGC-3’, 5’-GAGCAACTGCGCTCGGCCGAT-3’, 5’-CAAGCTTGAGCAGAACATGTT-3’, 5’-CACGAGCCAAGAGTGAGAAGAAC-3’, 5’-CTGACGGAAGTGACTCGG-3’, 5’-CTTGCTGATCAAGATGATG-3’; introduced oligonucleotide exchanges are underlined) were used in combination with either Universal or Reverse Primer to construct mutants T7S, R50H, N52S, F78C, S80L, M283V, T307I respectively using plasmid pUCCPA (1 ng) as template. Amplification products were restricted with SacI and PstI, and cloned in pUCCPA digested with the same endonucleases yielding the corresponding pUCCPA-derivatives. All constructs were verified by DNA sequencing using an ABI PRISM 310 Genetic Analyzer (Applied Biosystems). Mutant genes were subcloned into pGAL9 following the same procedure described above for the wild type gene to obtain the series of vectors named pGCCPA-T7S to pGCCPA-T307I (Table 1).
Construction of pDccpA.
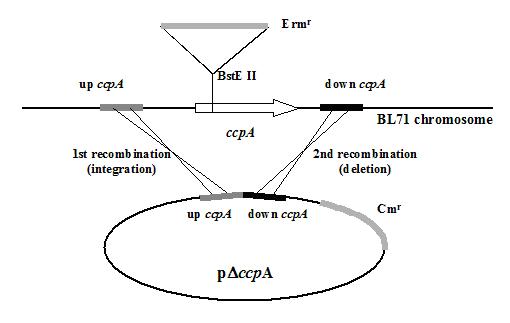
pCCPA2.6 containing a 2.6 kb insert carrying the L. casei ccpA gene and surrounding chromosomal DNA (Monedero et al., 1997) was amplified by reverse PCR using oligonucleotides dCCPA1 (5’-GAAGATCTCCGCCTTTTTCAGAAAGCC-3’) and dCCPA2 (5’-GAAGATCTCGAATTGTCAAACTAAGTGC-3’) introducing BglII restriction sites (underlined). The PCR product was digested with BglII, ligated, an used to transform E. coli. The derived vector contained a DNA fragment that excluded ccpA joining the regions upstream and downstream of ccpA (700 bp each). This fragment was isolated by restriction with SalI/XbaI and ligated to SalI/XbaI-digested pUCm1 (Monedero et al., 1997), yielding plasmid pDccpA (figure1).
Western blot analysis.
All L. casei strains were grown in MRS medium (Oxoid). Cells were harvested at an OD550 of 0.8 by centrifugation and cell extracts were subsequently prepared by disrupting cells with glass beads as described previously (Monedero et al., 1997). Proteins of cell extracts were separated by sodium dodecyl-sulfate-polyacrylamide gel electrophoresis on a 7.5% polyacrylamide gel and transferred to a nitrocellulose membrane (Trans-blot, Bio-Rad) by electroblotting. CcpA was detected with a rabbit polyclonal antiserum raised against CcpA of B. megaterium (Küster et al., 1996). CcpA antibodies on the membrane were visualized using anti-rabbit IgG conjugated to alkaline phosphatase (Roche) and BCIP/NBT chromogenic substrate (Sambrook et al., 1989).
Enzymatic assays.
N-acetyl-glucosaminidase activity measurements of L. casei cells grown on MRS basal medium supplemented with glucose or ribose were carried out as previously described (Monedero et al., 1997).
RESULTS:
Deletion of the L. casei ccpA gene.
The construction of a strain carrying a complete deletion of ccpA was required to study the effect of CcpA mutations in L. casei, avoiding undesired recombinations between mutant ccpA genes and the chromosomal fragments, which could reconstitute the wild type.
The deletion of ccpA required a process that involved two steps of a Campbell-like recombination followed by selection of the appropriate recombinants. For this purpose L. casei BL71 carrying an inactive ccpA gene disrupted by an erythromycin resistance cassette was transformed with the integrative plasmid pDccpA. One Err Cmr transformant was selected that had undertaken recombination upstream of ccpA; as revealed by Southern blot analysis (not shown). This transformant was grown in antibiotic-free medium for 50 generations. Strains that underwent a second recombination event downstream of ccpA were selected as Ers Cms colonies on MRS-agar replica plates. The genetic structure of one of these colonies (strain BL190) was analysed by PCR and dot blot to ensure a complete deletion of the ccpA gene (data not shown and Figure 1). BL190 is the first food-grade strain of lactobacilli with a deletion of the ccpA gene.
Mutagenesis of ccpA and expression of CcpA variants in BL190.
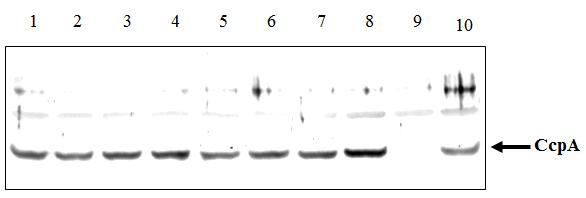
To gain molecular information on CcpA of L. casei and to possibly dissect CCR from growth function, mutations leading to single amino acid mutants were introduced in L. casei ccpA as described in Materials and Methods (Table 1). Positions for mutagenesis were chosen following detailed studies on CcpA of B. megaterium, where amino acid residues affecting either CCR and/or growth had been previously identified (Küster et al.,1999a; Küster et al.,1999b). The ccpA deleted strain, BL190, was transformed by electroporation with pGAL9 derivatives containing the wild type and the collection of ccpA mutants obtained. The resultant set of BL190 derivatives expressing ccpA (wild type and mutants) was used to study the effect of CcpA mutations on CCR (N-acetyl-glucosaminidase activity) and growth rate. To assure that the differences found were due to the mutations in CcpA and not merely to different expression levels or stability of the mutant proteins, a Western blot analysis of the different transformants was performed. As depicted in Figure 2, a band corresponding to L. casei CcpA could be clearly detected, although other bands appeared as unspecific reaction of the polyclonal antibodies used (Küster et al., 1996). The amount of CcpA protein in all mutants was very similar and also similar to the amount of wild-type CcpA expressed from pGCCPA. This result was therefore considered a valid control to analyse the phenotype of the mutants in further experiments. Expression of CcpA from pGAL9 derivatives was slightly higher than expression from the chromosomal copy (Figure 2).
Complementation of CCR by ccpA wild-type and mutant genes.
The effect of ccpA mutations on CCR was studied measuring N-acetyl-glucosaminidase activities in cells grown on glucose (repressing conditions) or ribose (non-repressing conditions). This activity has been shown to be a reliable reporter of the CCR status in L. casei (Monedero et al., 1997; Dossonnet et al., 2000; Viana et al., 2000). Table 2 shows N-acetyl-glucosaminidase activities of BL190 strains carrying plasmids that expressed either wild-type or mutant CcpA (T7S to T307I), as well as strains BL23 (wild type) and BL190 (DccpA). In the wild-type strain BL23, N-acetyl-glucosaminidase activity was repressed by glucose by a factor of 14 (activity on ribose/activity on glucose). Ribose-grown cells of BL190 (DccpA) had the same activity as ribose-grown cells of BL23, whereas in glucose-grown cells the activity was seven times higher than in the wild type rendering a CcpA-independent CCR of two fold.
Strain BL190 [pGCCPA] that expressed the wild-type CcpA was considered as reference to compare the behaviour of L. casei BL190 transformed with the different mutant derivatives of ccpA. BL190 [pGCCPA] showed 30-35% lower activity on glucose and ribose than the wild type BL23, possibly as consequence of CcpA overexpression. However, CCR was clearly restored, as the repression ratio was the same as in the wild type (14-fold). All the mutant variants of ccpA in BL190 displayed similar activities on ribose, but differences could be detected when grown on glucose. Strains BL190 [pGCCPA-T7S] and BL190 [pGCCPA-N52S] showed a partial, but significant de-repression. In these strains, N-acetyl-glucosaminidase activities of glucose-grown cells were four and five times greater, respectively, than the activity of the control. Glucose grown cells of BL190 [pGCCPA-S80L] and BL190 [pGCCPA-T307I] showed a N-acetyl-glucosaminidase activity below the detection level, indicating a significant hyper-repression in these two strains. It should be noticed that the two mutations showing a de-repressed phenotype are located in the N-terminal DNA binding domain as is the case in B. megaterium (Küster et al.,1999a), while the two mutations with hyper-repressed phenotype are found in the C-terminal corepressor binding domain and result in constitutive repression of the xyl promoter in B. megaterium (Küster et al.,1999b).
Table 2. N-acetyl-glucosaminidase activity
Strain | Activity* of glucose-grown cells | Activity* of ribose-grown cells | Repression factor |
BL190 [pGCCPA-T7S] | 14.7 ± 1.4 | 61.2 ± 10.5 | 4.1 |
| BL190 [pGCCPA-R50H] | 6.7 ± 3.0 | 55.0 ± 8.1 | 8.2 |
| BL190 [pGCCPA-N52S] | 19.9 ± 2.0 | 59.4 ± 6.9 | 3.0 |
| BL190 [pGCCPA-F78C] | 4.5 ± 1.1 | 35.8 ± 11.0 | 7.9 |
| BL190 [pGCCPA-S80L] | N. D. A. | 50.2 ± 17.4 | § |
| BL190 [pGCCPA-M283V] | 3.1 ± 0.4 | 55.1 ± 14.6 | 18.0 |
| BL190 [pGCCPA-T307I] | N. D. A. | 40.8 ± 14.1 | § |
| BL190 [pGCCPA] | 3.7 ± 0.2 | 50.3 ± 12.5 | 13.6 |
| BL190 (DccpA) | 38.3 ± 2.9 | 78.0 ± 6.0 | 2.0 |
| BL23 (wt) | 5.3 ± 0.1 | 76.9 ± 14.1 | 14.4 |
* Activity is given in nanomoles of p-nitrophenol released per minute per miligram of dry weight.
N. D. A. = no detectable activity.
§ = no detectable activity on glucose, repression factor tends to infinite.
Growth effect of ccpA mutations.
In addition to the effect on CCR, deletion of ccpA also resulted in a reduced growth rate. In order to study the influence of CcpA mutations on this phenotype, the doubling times of strains BL23, BL190, and BL190 carrying the plasmids expressing mutant CcpAs (T7S to T307I) and the wild-type protein were determined on MRS basal medium plus 0.5% glucose (Table 3). The doubling time of BL23 was 93 minutes, and when ccpA was deleted (BL190) the doubling time increased up to 118 minutes. However, the generation time of the wild-type was not restored when BL190 was transformed with pGCCPA. This growth restriction may be due to the expression of CcpA, to the presence of the plasmid pGCCPA, or derivatives, or to the use of erythromycin in the culture medium. The determination of the growth rate of L. casei carrying the same plasmid without ccpA such as pGAL9, could help to discriminate its cause. The doubling time of BL23 [pGAL9] was 126 min, very similar to BL190 [pGCCPA], suggesting that the presence of ccpA in multicopy might not be the factor affecting growth. Therefore, the doubling time of BL190 [pGCCPA] should be taken as a reference. All the mutants grew slower than the wild type, however, differences in doubling times were always below 13.5% (BL190 [pGCCPA-F78C]). Thus, the growth defect was not linked to the CCR phenotype as it was observed in the respective B. megaterium CcpA mutants.
Table 3. Doubling time of L. casei wt and ccpA mutants on glucose.
Strain | t2 (min) | Increment in t2 (%) |
| BL190 [pGCCPA-T7S] | 124.1 ± 1.4 | 4.6* |
| BL190 [pGCCPA-R50H] | 127.1 ± 3.2 | 7.2* |
| BL190 [pGCCPA-N52S] | 131.8 ± 2.2 | 11.1* |
| BL190 [pGCCPA-F78C] | 134.7 ± 1.6 | 13.5* |
| BL190 [pGCCPA-S80L] | 120.7 ± 0.0 | 1.8* |
| BL190 [pGCCPA-M283V] | 128.0 ± 0.3 | 7.9* |
| BL190 [pGCCPA-T307I] | 126.0 ± 1.6 | 6.2* |
| BL190 [pGCCPA] | 118.6 ± 1.8 | 27.0§ |
| BL190 (DccpA) | 118.3 ± 0.1 | 26.7§ |
| BL23 [pGAL9] | 126.0 ± 4.0 | 34.9§ |
| BL23 (wt) | 93.4 ± 0.8 |
* Increment in doubling time of the mutants compared to BL190 [pGCCPA]
§ Increment in doubling time of pGAL9 and pGCCPA transformed strains compared to BL23
DISCUSSION:
CcpA belongs to the LacI/GalR family of regulatory proteins (Weikert & Adhya, 1992). The crystal structure of the family member PurR (Schumaker et al., 1994) revealed three functional units, an N-terminal DNA binding domain (DBD) that contains a helix-turn-helix (HTH) DNA-binding motif, a C-terminal co-repressor binding domain (CBD) and a “hinge” helix joining both domains, which can be considered to be a functional part of the DBD, since it interacts with the DNA minor grove (Schumaker et al., 1994). The helices of the DBD are extremely sensitive to mutations, which usually result in a repressor protein defective in operator binding (Gordon et al., 1988; Kleina et al., 1990). Mutations T7S and N52S in L. casei CcpA are located in the positioning helix of the HTH motif and in the hinge helix, respectively (Figure 3). These mutations in the DBD show the same behaviour as the corresponding mutations in B. megaterium CcpA, which is a lack (or partial relief) of CCR when grown on the repressing carbon source glucose (Küster et al., 1999a).
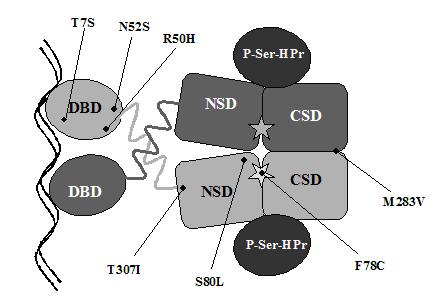
The CBD is structurally related to bacterial periplasmic binding proteins (Müller-Hill, 1983) and contains two topologically similar subdomains. Each CBD subdomain consists of a parallel b-strand core flanked by a-helices. Three crossover regions connect the two subdomains allowing relative movements between them upon co-repressor association and dissociation (Sharff et al., 1992; Olah et al., 1993). The CBD seems to be less sensitive to mutations than the DBD, although regions involved in subunit aggregation and sugar binding seem to be very sensitive within. Mutations F77C, M282V and T306I in the CBD of B. megaterium CcpA all have the same effect, which is a glucose-independent CCR, leading to permanent repression of xylose utilisation genes (Küster et al., 1999b). Surprisingly, none of the equivalent CDB mutations in L. casei CcpA (F78C, M283V and T307I) had similar effects. In the E. coli lactose repressor protein LacI, the co-repressor binding pocket is located between both CBD subdomains. This binding pocket establishes a number of hydrogen bonds (Asn246, Arg197 and Asp149) and a hydrophobic surface (Leu73, Ala75, Pro76, Ile79, Trp220 and Phe293) to interact with the correpressor IPTG (Lewis et al., 1996). Mutations F78C and S80L in L. casei CcpA are in the proximity of the co-repressor binding pocket (Figure 3). While F78C had no effect on CCR, S80L showed hyper-repression in the presence of glucose but no effect on ribose. The exchange of serine for leucine could improve the hydrophobic environment of the co-repressor binding pocket leading to an enhanced and more stable binding of the co-repressor and therefore more efficient CcpA activity, which would occur only in the presence of glucose. The M283 analogous residue in LacI and PurR (Tyr282) plays a role in the dimerisation of the C-terminal subdomains of the protein core (Schumaker et al., 1994; Friedman et al., 1995). In L. casei CcpA, mutation M283V had no effect on CCR although this mutation results in constitutive CCR in B. megaterium (Küster et al.,1999b). Like S80L, T307I showed hyper-repression in the presence of glucose, whereas this mutation in B. megaterium CcpA causes permanent CCR (Küster et al.,1999b). This threonine residue is conserved among CcpA-like proteins but not among other members of the LacI-GalR family (Kraus et al., 1998). Only one protein of the CcpA subfamily, RegA from Clostridium acetobutilicum, carries an isoleucine at this position. It has been shown that RegA complements a B. subtilis ccpA mutant leading to constitutive repression of amyE (Davison et al., 1995).
Interestingly none of the CBD mutations in L. casei CcpA had the same effect as the corresponding mutations in B. megaterium CcpA, for the reporters assayed. From these results, it can be inferred that, although CCR signal response is efficiently fulfilled by both bacteria, the residues involved and molecular changes in CcpA caused by interactions with the cofactor(s) could be different in both species. In a previous work, remarkable structural differences between B. megaterium and L. casei CcpA were suggested by the inefficient interaction of L. casei CcpA with the Bacillus megaterium P-Ser-HPr, as determined by surface plasmon resonance and lack of CCR complementation by L. casei ccpA in a B. megaterium DccpA strain (Mahr et al., 2002). However, it has been described that in B. subtilis there is a large number of genes under CCR which show a different response to the inactivation of ccpA (Moreno et al., 2001), therefore, it could be conceivable that CcpA mutations, also in L. casei, could have a different effect on other promoters.
The growth effect of specific mutations was shown to be completely independent from the CCR of N-acetyl-glucosaminidase activity. Mutation F78C showed the highest increase in doubling time and it was not affected in CCR. The two hyper-repressing mutations, S80L and T307I, had a slightly different behaviour with respect to growth. S80L showed almost normal growth, while T307I had small growth defect (6% increase in doubling time). The three mutations in the DBD subdomain, two of them with a de-repressed phenotype, differed in their growth capabilities, following the increasing order: N52S < R50H < T7S.
In summary, results obtained in this work suggest that CCR and growth defect phenotypes of CcpA mutants might not be linked in L. casei as they possibly are in B. megaterium and, that conserved amino acid residues in equivalent positions may not be playing the same role in B. megaterium and L. casei. Two mutations leading to a CCR hyper-repressing phenotype were identified. Up to date such phenotype had never been reported constituting an excellent starting point for further studies. In particular, future works should analyse the effect of these mutations on CCR / CCA on a global basis or, at least, of a larger number of genes.
ACKNOWLEDGMENTS:
C.D.E. and K.M. contributed equally to this work. We thank Elke Küster-Schöck for the gift of anti-CcpA antibodies. This work was financed by the EU project BIO4-CT96-0380 and by funds of the Spanish CICyT (Interministerial Commission for Science and Technology) (Ref. ALI 98-0714). C.D.E was the recipient of a fellowship from the Spanish Government. K. M. was supported by the Graduiertenkolleg Kontrolle der RNA-Synthese of the Deutsche Forschungsgemeinschaft.
REFERENCES:
Aung-Hilbrich, L. M., Seidel, G., Wagner, A. & Hillen, W. (2002) Quantification of the influence of HPrSer46P on CcpA-cre interaction. J Mol Biol 319, 77-85.
Brückner, R. & Titgemeyer, F. (2002). Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol Lett 209,141-148.
Davison, S. P., Santangelo, J. D., Reid, S. J. & Woods, D. R. (1995). A Clostridium acetobutylicum regulator gene (regA) affecting amylase production in Bacillus subtilis. Microbiology 141, 989-996.
Deutscher, J., Küster, E., Bergstedt, U., Charrier, V. & Hillen, W. (1995). Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in gram-positive bacteria. Mol Microbiol 15, 1049-1053.
Dossonnet, V., Monedero, V., Zagorec, M., Galinier, A., Pérez-Martínez, G. & Deutscher, J. (2000). Phosphorylation of HPr by the bifunctional HPr Kinase/P-ser-H phosphatase from Lactobacillus casei controls catabolite repression and inducer exclusion but not inducer expulsion. J Bacteriol 182, 2582-2590.
Egeter, O. & Brückner, R. (1996). Catabolite repression mediated by the catabolite control protein CcpA in Staphilococcus xylosus. Mol Microbiol 15, 1049-1053.
Faires, N., Tobisch, S., Bachem, S., Martin-Verstraete, I., Hecker, M. & Stülke, J. (1999). The catabolite control protein CcpA controls ammonium assimilation in Bacillus subtilis. J Mol Microbiol Biotechnol 1, 141-148.
Friedman, A. M., Fischmann, T. O. & Steitz, T. A. (1995). Crystal structure of lac repressor core tetramer and its implications for DNA looping. Science 268, 1721-1727.
Fujita, Y., Miwa, Y., Galinier, A. & Deutscher, J. (1995). Specific recognition of the Bacillus subtilis gnt cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorilated HPr. Mol Microbiol 17, 953-960.
Giammarinaro, P. & Paton, J. C. (2002) Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect Immun 70, 5454-5461.
Gordon, A. J., Burns, P. A., Fix, D. F., Yatagai, F., Allen, F. L., Horsfall, M. J., Halliday, J. A., Gray, J., Bernelot-Moens, C. & Glickman, B. W. (1988). Missense mutation in the lacI gene of Escherichia coli. Inferences on the structure of the repressor protein. J Mol Biol 200, 239-251.
Gosseringer, R., Küster, E. Galinier, A., Deutscher, J. & Hillen, W. (1997). Cooperative and non-cooperative DNA binding modes of catabolite control protein CcpA from Bacillus megaterium result from sensing two different signals. J Mol Biol 266, 665-676.
Hueck, C. J., Kraus, A., Schmiedel, D. & Hillen, W. (1995). Cloning, expression and functional analyses of the catabolite control protein CcpA from Bacillus megaterium. Mol Microbiol. 16, 855-864.
Jones, B. E., Dossonnet, V., Küster, E., Hillen, W., Deutscher, J. & Klevit, R. E. (1997). Binding of the catabolite repressor protein CcpA to its DNA target is regulated by phosphorilation of its corepressor HPr. J Biol Chem 272, 26530-26535.
Kim J.H., Voskuil, M. I. & Chambliss, G. H. (1998). NADP, corepressor for the Bacillus catabolite control protein CcpA. Proc Natl Acad Sci U S A 95, 9590-9595.
Kim, H. J., Roux, A. & Sonenshein, A. L. (2002) Direct and indirect roles of CcpA in regulation of Bacillus subtilis Krebs cycle genes. Mol Microbiol 45, 179-190.
Kleina, L. G. & Miller, J. H. (1990). Genetic studies of the lac repressor. XIII. Extensive amino acid replacements generated by the use of natural and synthetic nonsense suppressors. J Mol Biol 212, 295-318.
Kraus, A., Küster, E., Wagner, A., Hoffmann, K. & Hillen, W. (1998). Identification of a co-repressor binding site in catabolite control protein CcpA. Mol Microbiol 30, 955-963.
Küster, E., Luesink, E. J., de Vos, W. M. & Hillen, W. (1996). Immunological crossreactivity to catabolite control protein CcpA from Bacillus megaterium in found in many Gram-positive bacteria. FEMS Microbiol Lett 139, 109-115.
Küster, E., Hilbich, T., Dahl, M. K. & Hillen, W. (1999a). Mutations in catabolite control protein CcpA separating growth effects from catabolite repression. J Bacteriol 181, 4125-4128.
Küster, E., Wagner, A., Völker, U. & Hillen, W. (1999b). Mutations in catabolite control protein CcpA showing glucose-independent regulation in Bacillus megaterium. J Bacteriol 181, 7634-7638.
Landt, O., Grunert, H. P. & Hahn, U. (1990). A general method for rapid site-directed mutagenesis using the polymerase chain reaction. Gene 96, 125-128.
Leboeuf, C., Leblanc, L., Auffray, Y. & Hartke, A. (2000). Characterization of the ccpA gene of Enterococcus faecalis: identification of starvation-inducible proteins regulated by ccpA. J Bacteriol 182, 5799-5806.
Lewis, M., Chang, G., Horton, N. C., Kercher, M. A., Pace, H. C., Schumacher, M. A., Brennan, R. G. & Lu, P. (1996). Crystal structure of the lactose operon repressor and its complexes with DNA and inducer. Science 271, 1247-1254.
Lokman, B. C., Heerikhuisen, M., Leer, R. J., van den Broek, A., Borsboom, Y., Chaillou, S., Postma, P. W., & Pouwels, P. H. (1997). Regulation of expression of the Lactobacillus pentosus xylAB operon. J Bacteriol 179, 5391-5397.
Ludwig, H., Homuth, G., Schmalisch, M., Dyka, F. M., Hecker, M. & Stülke, J. (2001). Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon. Mol Microbiol 41, 409-422.
Ludwig, H., Meinken, C., Matin, A. & Stülke, J. (2002). Insufficient expression of the ilv-leu operon encoding enzymes of branched-chain amino acid biosynthesis limits growth of a Bacillus subtilis ccpA mutant. J Bacteriol 184, 5174-5178.
Luesink, E. J., van Herpen, R. E., Grossiord, B. P., Kuipers, O. P. & de Vos, W. M. (1998). Transcriptional activation of the glycolytic las operon and catabolite repression of the gal operon in Lactococcus lactis are mediated by the catabolite control protein CcpA. Mol Microbiol 30, 789-798.
Mahr, K., Esteban, C. D., Hillen, W., Titgemeyer, F. & Pérez-Martínez, G. (2002). Cross communication between components of carbon catabolite repression of Lactobacillus casei and Bacillus megaterium. J Mol Microbiol Biotechnol. 4, 489-494.
Miwa, Y., Saikawa, M. & Fujita, Y. (1994). Possible function and some properties of the CcpA protein of Bacillus subtilis. Microbiology 140, 2567-2575.
Monedero, V., Gosalbes, M.J. & Pérez-Martínez, G. (1997). Catabolite repression in Lactobacillus casei ATCC 393 is mediated by CcpA. J Bacteriol 179, 6657-6664
Moreno, M. S., Schneider, B. L., Maile, R. R., Weyler, W. & Saier, M. H. Jr. (2001). Catabolite repression mediated by the CcpA protein in Bacillus subtilis: novel modes of regulation revealed by whole-genome analyses. Mol Microbiol 39, 1366-1381.
Müller-Hill, B. (1983). Sequence homology between Lac and Gal repressors and three sugar-binding periplasmic proteins. Nature 302, 163-164.
Olah, G. A., Trakhanov, S., Trewhella, J. & Quiocho, F. A. (1993). Leucine/isoleucine/valine-binding protein contracts upon binding of ligand. J Biol Chem 268, 16241-16247.
Pérez-Martínez, G., Kok, J., Venema, G., Van Dijl, J. M., Smith, H. & Bron, S. (1992). Protein export elements from Lactococcus lactis. Molec Gen Genet 234, 401-411.
Posno, M., Leer, R. J., van Luijk, N., van Gienzen, M. J. F., Heulvelmans, P. T. H. M., Lokman, B. C. & Powels, P. H. (1991). Incompativility of Lactobacillus vectors with replicons derived from small cryptic Lactobacillus plasmids and segregational instability of the induced vectors. Appl Environ Microbiol 57, 1822-1828.
Sambrook, J., Fritsch, E. F. & Maniatis, T. (1989). Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N. Y.
Schumaker, M. A., Choi, K. Y., Zalkin, H. & Brennan, R. G. (1994). Crystal structure of LacI member, PurR, bound to DNA: minor groove binding by a helices. Science 266, 763-770.
Sharff, A. J., Rodseth, L. E., Spurlino, J. C. & Quiocho, F. A. (1992). Crystallographic evidence of a large ligand-induced hinge-twist motion between the two domains of the maltodextrin binding protein involved in active transport and chemotaxis. Biochemistry 31, 10657-10663.
Stanley, N. R., Britton. R. A., Grossman. A. D. & Lazazzera, B. A. (2003) Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. J Bacteriol 185, 1951-1957.
Titgemeyer, F. & Hillen, W. (2002). Global control of sugar metabolism: a gram-positive solution. Antonie Van Leeuwenhoek 82,59-71
Turinsky, A. J., Moir-Blais, T. R., Grundy, F. J. & Henkin, T. M. (2000). Bacillus subtilis ccpA gene mutants specifically defective in activation of acetoin biosynthesis. J Bacteriol 182, 5611-5614.
Viana, R., Monedero, V., Dossonnet, V., Vadeboncoeur, C., Pérez-Martínez, G. & Deutscher, J. (2000). Enzyme I and HPr from Lactobacillus casei: their role in sugar transport, carbon catabolite repression and inducer exclusion. Mol Microbiol 36, 570-584.
Wen, Z. T. & Burne, R. A. (2002) Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl Environ Microbiol. 68, 1196-1203.
Weikert, M. J. & Adhya, S. (1992). A family of bacterial regulators homologous to Gal and Lac repressors. J Biol Chem 267, 15869-15874.

